 | 
Objections to Evolutionary Theories of the Origin of Life (Sidebar 1)

The common evolutionary scenario for the origin of life is the "pre-biotic soup" from which the first biological molecules spontaneously formed and aggregated to form polymers, macromolecules, protocells, and finally, true cells. In the first stage, the atmosphere contained no oxygen, and was very reducing. Three and a half billion years ago, the oceans cooled. Without an ozone layer in the atmosphere, and with lightning as an energy source, ultraviolet light reacted with ammonia, methane, hydrogen, carbon monoxide, carbon dioxide, and nitrogen in the atmosphere to form the first amino acids, nucleic acids and sugars. The molecules were washed to the ocean in rain where they accumulated into a thick "soup." As the molecules collected to a sufficient concentration, they polymerized to form chains of DNA and RNA, polypeptides (short protein chains), and fatty acids. The fatty acids formed membranes and began to enclose the organic molecules into protocells. Eventually the polypeptides became more complex and acquired enzymatic abilities and the protocells became true cells.
Below are five, out of many, objections to the evolutionary theories of the origin of life.
1. Polymerization of Macromolecules—polymerization is the formation of a macromolecule by joining small molecules together with covalent bonds. In the cell there are four important types of polymers: proteins which are made of amino acids, nucleic acids which are made of nucleotides, polysaccharides which are made of sugars, and lipids which are made of fatty acids. There is nothing intrinsic about the subunits (i.e. small molecules) themselves that directs correct polymerization of the correct molecules in the correct positions. In cells, polymerization requires an energy source, protein enzymes to direct the process, and a supply of subunits. Following is an example that demonstrates the difficulty of correct, spontaneous nucleic acid polymerization.
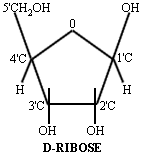 
The drawings represent the pentose (five carbons in a ring) sugar "ribose" on the left and the base Adenine on the right. The five carbon (C) atoms in ribose and the carbon and nitrogen (N) atoms in adenine are numbered in the conventional fashion to facilitate discussion. (A corner without a letter is understood to be carbon.) Black lines represent single covalent bonds and blue lines represent double covalent bonds. Ribose is part of the backbone of RNA. Deoxy-ribose, the sugar in DNA, is identical to ribose except that the 2' Carbon has a hydrogen atom in the place where the hydroxyl (OH) is. Ribose has five locations where other molecules can bind: alpha 1', beta 1', 2', 3', and 5'. All five of these locations have a hydroxyl group, and other molecules can form bonds here.
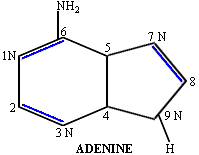
Adenine has three isomeric forms (i.e. the same atoms are found in different locations) because the NH2 can bind at the C2, C6, or the C9 position. Only the C6 position is found in cells. Ribose also has isomers. By rearranging the hydrogen atoms and the hydroxyl groups associated with positions C2' and C3', eight different isomers of ribose can be formed, however they have other names (e.g. xylose, lyxose, and arabinose). Only ribose is found in DNA and RNA.
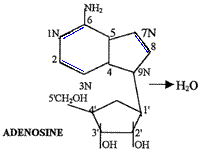
The first step in forming a polymer of nucleotides is the formation of a nucleoside. To form a nucleoside, the base (e.g. adenine) binds to the sugar (ribose or deoxyribose) in a specific location. The 9N of adenine binds to the beta-1'C of (deoxy-) ribose. This is the only correct location of the bond, but two other sites on adenine (N7 and NH2) can also bind to any of the other four hydroxyl groups on ribose (alpha-1', 2', 3', or 5'). The drawing represents the nucleoside adenosine.
So far, there are 360 different combinations of adenosine:
3 isomers of adenine X 8 arrangements of ribose X 3 locations for bond formation on adenine X 5 locations for bond formation on ribose = 360.
After forming nucleosides, the next step in polymerizing DNA and RNA is the formation of nucleotides.
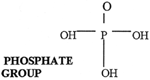
Nucleotides are nucleosides with a phosphate group attached to the 5' C of the sugar. The phosphate could, however, also attach to the 2'C or to the 3' C of the sugar. Now there are (360 X 3) = 1,080 possible arrangements for the nucleotide formation, however, only one is correct. To begin polymerization, two nucleotides must join together.
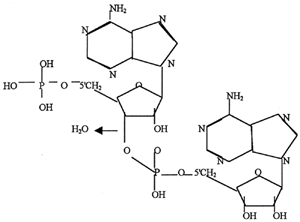
In the diagram, two adenine nucleotides are joined together to form a dinucleotide. The second adenine binds to the phosphate of the first adenine by the 3'C. This is the only correct location for binding, however, the second nucleotide could have joined at one of two hydroxyl groups, so the total number of ways in which adenine can form a dinucleotide is 1,080 X 2 = 2,160. The demand for exact bonding and the vast number of sites where random bonding can occur makes polymerization of a meaningful strand of nucleic acids extremely difficult without the direction of a previously existing system of proteins.
2. Hydrolysis—Hydrolysis is the splitting of a compound with the breakdown of a water molecule. Due to hydrolysis, the half-life of polymers of amino acids, nucleic acids, sugars, and fats in a water solution is days or maybe up to months. The pH of a solution influences what types of organic compounds are hydrolyzed. For example, in an acidic solution, the amino acid tryptophan would be hydrolyzed rapidly, as would serine and threonine be to an extent. Other amino acids cysteine, glutamine, and asparagine would be altered. In an alkaline solution (which the primitive ocean was supposed to have been), serine, threonine, cystine, cysteine, and arginine would have been broken apart and several others modified.
3. Ultra-violet destruction—The supposed conditions of the primordial atmosphere do not include oxygen, so there would have been no ozone layer to filter the ultra-violet light (UV) from the sun. UV would have had the following deleterious effects on chemical evolution:

| a.) UV would have polymerized most of the methane in the atmosphere. Polymerized methane products are hydrocarbons and fatty acids, and they would have fallen from the atmosphere and collected in the ocean.
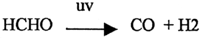
b.) UV would have broken down formaldehyde to carbon monoxide and hydrogen by the process of photolysis.
c.) Carbon monoxide in the alkaline ocean would have been irreversibly converted to formate.
d.) Ammonia (NH3) is necessary in chemical evolution for the formation of amino acids. However, in the atmosphere, ammonia would be broken down to nitrogen and hydrogen very quickly by photolysis, and very little ammonia would remain in the atmosphere.
e.) Hydrogen sulfide would also have been photolyzed to hydrogen gas and sulfur in 10,000 years or even less.
f.) UV would also have broken down water vapor to oxygen and hydrogen. Some experiments suggest that photolysis of water vapor by UV would have generated a significant amount of oxygen in the atmosphere.
g.) As UV enters the Earth's atmosphere, methane—even at low concentrations as it is in today's atmosphere—absorbs all short-wavelength UV radiation that reaches 30 kilometers in altitude. Any amino acids or other organic molecules that formed in the atmosphere by UV photolysis would have had to be synthesized in the upper atmosphere. It has been estimated that the amount of time for organic molecules to descend from the upper atmosphere to the Earth is up to three years. Therefore, the molecules would have been exposed to destructive long-wave UV for up to three years. UV could still affect those organic molecules that did survive to the ocean until they had reached a depth of some tens of meters.
|
4. Chirality— Chiral molecules are those which have a mirror image of themselves. The two mirror image molecules have exactly the same atoms, but they are arranged in different orders. For example, the two molecules below are chiral, but are not the same.

Chiral molecules such as the above molecules are sometimes described as "right handed" and "left handed" or as optical isomers. (Note: even though the molecules appear that they could be rotated out of the 2 dimensional plane to be the same, the bonds would push the atoms into opposing conformations.) Many types of molecules have optical isomers, including amino acids. In nature, L-amino acids (or left-handed) and D-amino acids (or right-handed) are found equally, and will bond together without distinction; however, the functional proteins of nearly every type of cell are composed exclusively of L-amino acid (an exception is found in the capsule proteins of certain bacteria). The shape of a protein determines its ability to perform a function. If a protein incorporates a D-amino acid, the shape will be changed due to the improper conformation. Theories of chemical evolution have no solution to the problem of chiral amino acids being formed and subsequently binding together.
5. Holism (Irreducible complexity)—Dr. Michael Behe defines irreducibly complex as a "single system composed of several well-matched, interacting parts that contribute to the basic function, wherein the removal of any one of the parts causes the system to effectively cease functioning." For example, a conventional mousetrap has five main parts—the base, the hammer, the spring, the catch, and the holding bar. A mousetrap cannot work if even one of these parts is missing. Conversely, no one part, independent of the whole, has any value as a mouse catching device. In biological systems, many complex proteins, processes, and organelles are irreducibly complex. The examples used by Behe are cilia and flagella (which are used in some cells for movement), the blood clotting system, cellular transportation and the immune system.
References:
Behe, Michael J. Darwin's Black Box. Free Press, New York. 1996.
Thaxton, Charles B; Bradley, Walter L.; and Olsen, Roger L. The Mystery of Life's Origin. Lewis and Stanley Publishers, Dallas. 1984.
|


