
The composition of earth's atmosphere is vital for life on earth. In addition to providing life-sustaining gases, the elements and compounds in the atmosphere give us a clue in determining the age of the earth. Of primary importance in this discussion is helium.

Properties of Helium:
Helium is a stable, inert gas. It occurs in two isotope forms—3He and 4He.

Sources of Helium
1. Primordial Helium (helium found in earliest earth's crust):
 The evolutionary model predicts that there was no primordial helium because the atmosphere had yet to form. The evolutionary model predicts that there was no primordial helium because the atmosphere had yet to form.
 The creation model predicts that the early atmosphere was similar to what it is now, although the Flood may have caused significant changes. The creation model predicts that the early atmosphere was similar to what it is now, although the Flood may have caused significant changes.
2. Radioactive decay of Uranium-235 and -238 and of Thorium-232 results in formation of 4He as a byproduct.
3. 3He is released from the mantle into the ocean from which it escapes to the atmosphere. 3He is not a known by-product of radiometric decay, so it is assumed to be primordial in the mantle.

The Rate of Flow of Helium into the Atmosphere
Estimates have been made for the rate of flow of 4He and 3He into the atmosphere, though these estimates have several assumptions inherent to them. 4He has an estimated flow rate of 2 x 106 atoms/cm2/sec over the entire surface of the earth. 3He has an estimated flow rate of 4.6 atoms/cm2/sec.

Amount of Helium in Atmosphere
The total mass of 4He in the atmosphere is 6 x 1038 atoms. The total mass of 3He in the atmosphere is 1 x 1033 atoms.

Implications of amount found:
According to the long-age model, at a flow rate of 2 x 106 atoms/cm2/sec over 4.5 billion years, there should be about 2,000 times the amount of 4He that is actually measured. Therefore, some have suggested that helium must escape from the atmosphere.

Theories for the escape of helium from the atmosphere:
One theory that attempts to explain how and why helium escapes from the atmosphere is the thermal escape theory. The density of the atmosphere decreases with height. In the lower atmosphere, the particles are dense in concentration and collide frequently. They assume a Maxwellian distribution. The exosphere is the region in the upper atmosphere where collisions are negligible. Some particles from the base of the exosphere are "ejected upward with velocities less than that required for escape...and return to the base of the exosphere."[1]. A fraction of the ejected particles have velocity greater than the escape velocity and these particles are emitted into space. The rate of escape from the atmosphere has been estimated to be 5 x 104 atoms/cm2/sec.

Implications of the rate of helium escape from the atmosphere:
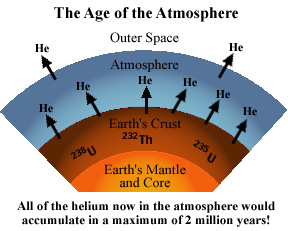 The estimated rate at which helium moves from the earth's crust to the atmosphere is 2 x 106 atoms/cm2/sec. The estimated rate of the escape of helium from the exosphere is 5 x 104 atoms/cm2/sec. At these rates, the amount of helium which is found in the atmosphere, starting with zero primordial helium, would take only 1.8 million years to accumulate. This is a real and acknowledged problem to those who believe that the earth formed 4.5 billion years ago. Other factors have been proposed for removing helium such as polar winds, solar wind sweeping, and hot-ion exchange, but none of these have been demonstrated to have significant effects. The estimated rate at which helium moves from the earth's crust to the atmosphere is 2 x 106 atoms/cm2/sec. The estimated rate of the escape of helium from the exosphere is 5 x 104 atoms/cm2/sec. At these rates, the amount of helium which is found in the atmosphere, starting with zero primordial helium, would take only 1.8 million years to accumulate. This is a real and acknowledged problem to those who believe that the earth formed 4.5 billion years ago. Other factors have been proposed for removing helium such as polar winds, solar wind sweeping, and hot-ion exchange, but none of these have been demonstrated to have significant effects.

An Alternative Theory:
Young earth creationists propose that the only problem with the amount of helium in the atmosphere is the assumption that the earth is 4.5 billion years old. According to the young-earth creation model, the earth is only thousands of years old (less than 10 thousand). In this model, nearly all of the helium in the atmosphere is primordial—placed in the atmosphere by God. Probably less than 1% of the total helium has been added by radioactive decay, unless there was some accelerated decay event in the early earth history. The flood of Noah's time may also have affected the amount of helium in the atmosphere if there was a water vapor canopy around the earth that collapsed.

|

